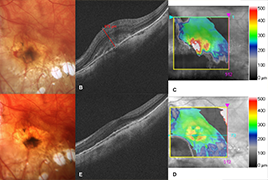
Purpose: To evaluate the safety and clinical efficacy of ranibizumab (Lucentis) in the treatment of choroidal neovascularization (CNV) caused by diseases other than age-related macular degeneration (AMD).
Patients: 21 patients with mean age 61 ± 17.2 years (min 16, max 85) with CNV due to causes other than AMD, in particular pathological myopia (n=11), angioid streaks (n=3), central serous chorioretinopathy (n=2), North Carolina macular dystrophy (n=1), dominant familial drusen (n=1) and idiopathic CNV (n=3).
Methods: The patients were treated at the Ophthalmology Department of the University Hospital in Hradec Kralove with three monthly initial intravitreal injections of ranibizumab 0.5 mg with subsequent treatment regimen pro re nata (PRN). The best corrected visual acuity (BCVA) was evaluated on the ETDRS optotypes (Early Treatment Diabetic Retinopathy Study), central retinal thickness (CRT) was measured by optical coherent tomography (OCT) (Zeiss, Cirrus). These parameters were evaluated before start of the study and then at 1 (BCVA only), 4, 8, and 12 months during treatment. We also evaluated the possible occurrence of ocular and systemic side effects.
Results: Statistically significant improvement in the mean of BCVA score of 11.4 letters (p<0.001) at the end of the follow-up period in patients with myopic CNV was observed. In the subgroup of CNV patients from other causes BCVA improvement was 5.9 letters (p=0.043). The mean CRT at 12 months statistically significantly decreased by 42.2 μm (p=0.028) in patients with myopic CNV and by 119.4 μm (p=0.002) in patients with CNV from other causes. We have not detected any serious ophthalmic or systemic side effects associated with ranibizumab s treatment.
Conclusions: Intravitreal ranibizumab given in the PRN regimen after the initial three monthly doses was demonstrated efficiency and safety in the treatment of CNV due to cause other than AMD during the annual follow-up.